Table of Contents
- – Buy [Pdf] The Acute Effects Of Cannabidiol On E...
- – Buy Clinical Trials - Parkinson's Disease And ...
- – Cannabis And Cannabinoids' Therapeutic Applica...
- – Buy Clinical Trial On Parkinson's Disease: Ca...
- – Buy Cbd Oil For Parkinson's Disease: Possible...
- – The Role Of Cannabidiol In Neurological Diso...
- – A0580 - Sigma-aldrich now available in LA
Buy [Pdf] The Acute Effects Of Cannabidiol On Emotional Processing And ... in TEXAS
The study was conducted in accordance with the Helsinki Declaration of 1994 and subsequent amendments. The trial was prospectively registered with the Australian New Zealand Clinical Trials Register (CT-2019-CTN-01224-1). Eligible participants were aged over 25 years with CNCP, on long-term treatment (at least 12 months) with high dose (oral morphine equivalent daily dose [OMEDD] ≥ 60 mg) opioid analgesia.

Participants were recruited at two sites, a public hospital pain management clinic and at a community medicinal cannabis clinic. Eligibility screening, informed consent, and baseline assessment were conducted by a member of the research team. Inclusions: aged over 25 years of age; patients with CNCP on long-term treatment (at least 12 months) receiving high dose (OMEDD > 60 mg) opioid analgesia; willing to cease driving a motor vehicle for the duration of the study; willing to undertake fasting requirements and meal restrictions whilst providing blood samples for pharmacokinetic studies; agree to undergo all assessments for trial duration of 5 weeks including blood testing for pharmacokinetic analysis; psychometrics and a saliva swab for a drug screen; no cannabis use in previous month, as confirmed by a negative drug screen.
Buy Clinical Trials - Parkinson's Disease And Movement Disorders ... in NYC - limited time only
Pharmacokinetic studies are typically conducted in healthy controls. Persons with CNCP often have multiple physical comorbidities and are prescribed multiple medications. This study was conducted in a “real world” cohort which necessitated decisions regarding limitations on prior and concomitant treatments and/or drug interactions. THC induces CYP1A2 activity and reduces serum concentrations of drugs metabolized by CYP1A2, e.
anti-psychotic medications such as clozapine, olanzapine, haloperidol, and chlorpromazine [20]. Thus persons with psychosis were excluded from this study. Serum concentrations of duloxetine and naproxen may be reduced with concomitant use of THC: persons on these drugs were eligible for recruitment but were required to be monitored for drug interactions.
Cannabis And Cannabinoids' Therapeutic Applications And Safety now in NC - limited time
Tramadol metabolism is primarily mediated through CYP2D6, CYP3A4, and CYP2B6 [21]. Carbamazepine is a potent inducer of CYP3A4, and CBD may inhibit its metabolism resulting in increased serum concentrations. Serum concentrations of topiramate have also been observed to rise with increasing CBD dose but changes have been reported to be within the accepted therapeutic range [22].

Current use of valproate was an exclusion for the current study as this combination may elevate aspartate transaminase (AST) and/or alanine aminotransferase (ALT) concentrations [23]. Benzodiazepines were excluded because of potential excessive sedation in combination with the investigational product (ZTL-103). No new medication other than the investigational product, opioid medications for CNCP, and contraceptives was permitted to be commenced after initiation on to the trial.
Buy Clinical Trial On Parkinson's Disease: Cannabidiol - Ich Gcp in NYC - limited time
The pharmacokinetics were investigated on day 1 after a single dose of 2. 5 mg THC/2. 5 mg CBD after fasting for 12 h, day 8 after a single dose of 2. 5 mg THC/2. 5 mg CBD following a high fat meal, day 15 after a single dose of 5 mg THC/5 mg CBD after twice daily 2.
5 mg CBD for 7 days, day 22 after a single dose of 7. 5 mg THC/7. 5 mg CBD after twice daily 5 mg THC/5 mg CBD for 7 days, and day 29 after a single dose of 12. 5 mg THC/12. 5 mg CBD after twice daily 7.
Buy Cbd Oil For Parkinson's Disease: Possible Treatment And ... in NC - limited time
5 mg CBD for 7 days. Participants returned for final assessments on day 36, after a 7-day washout period; however, no bloods were taken on this day as it was not anticipated that there would be any THC or CBD 1 week after a single dose of 12. 5 mg THC/12.
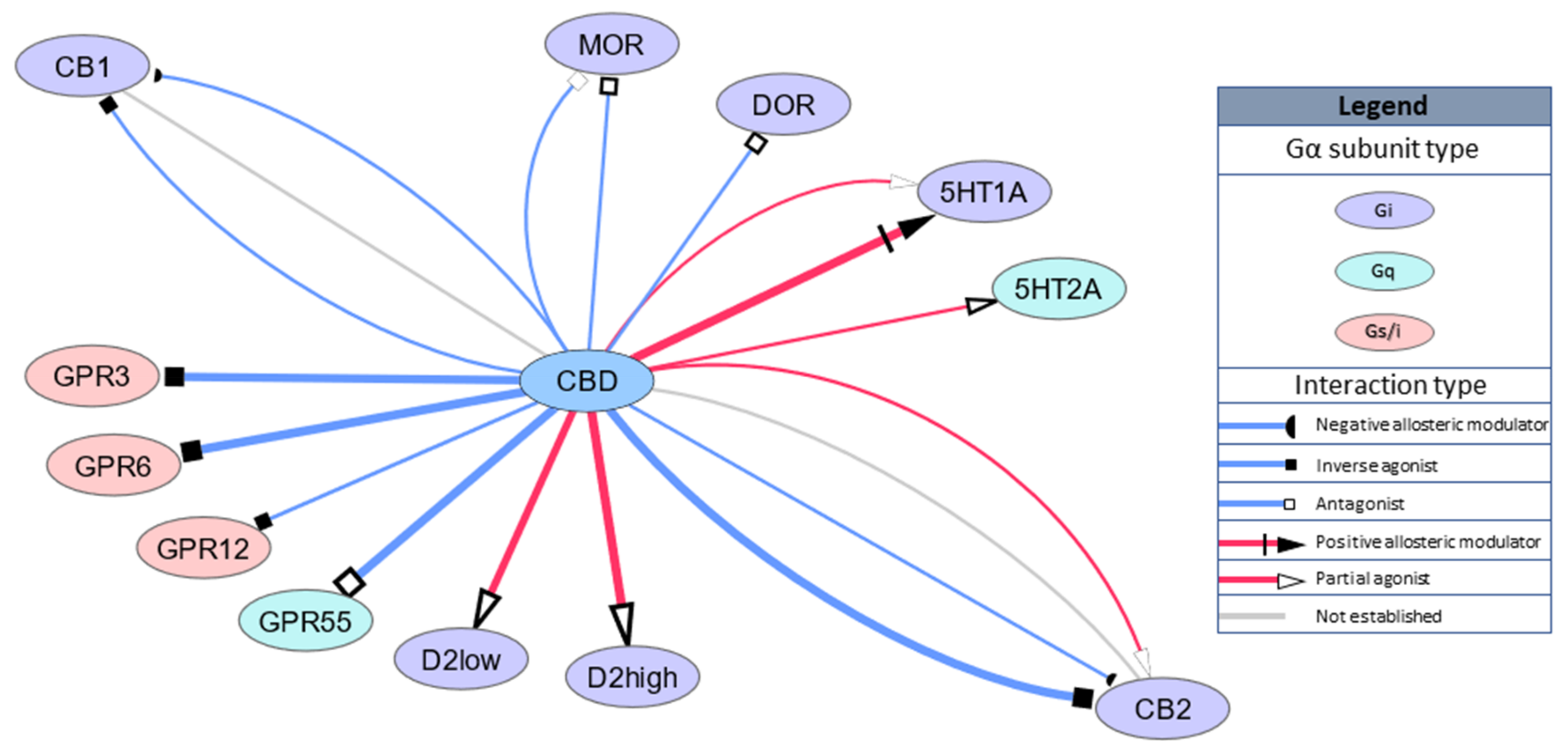
As an indication of this a single dose of 12. 5 mg THC/12. 5 mg CBD was given the previous evening at 4 pm prior to the day 29 pharmacokinetic (PK) analysis. The concentrations of THC and CBD in the predose sample approximately 20 h later were generally around 0.
The Role Of Cannabidiol In Neurological Disorders. - Abstract now in NC
Thus it would be highly unlikely that after a single dose of 12. 5 mg THC/12. 5 mg CBD there would be detectable THC and CBD 148 h later. To explore secondary objectives, participants completed the Brief Pain Inventory (BPI) [24] which has two subscales, namely pain severity and pain interference (i.
how pain impacts function and daily life), and recorded opioids, other analgesics, and any other medications, daily. At each visit to the study site (days 1, 8, 15, 22, 29, 36) participants completed the Depression, Anxiety, and Stress Scale (DASS-21) [25], Insomnia Severity Index (ISI) [26], and underwent a physical examination by a clinician.
A0580 - Sigma-aldrich now available in LA
All participants completed a daily sleep diary. Three variables were recorded: Sleep onset latency (SOL)—the participant’s perceived ease of falling asleep (Likert scale); Total sleep time (TST)—“Last night I slept a total of x hours”; Sleep quality—“When I woke up for the day I felt: refreshed, somewhat refreshed or fatigued”.
Table of Contents
- – Buy [Pdf] The Acute Effects Of Cannabidiol On E...
- – Buy Clinical Trials - Parkinson's Disease And ...
- – Cannabis And Cannabinoids' Therapeutic Applica...
- – Buy Clinical Trial On Parkinson's Disease: Ca...
- – Buy Cbd Oil For Parkinson's Disease: Possible...
- – The Role Of Cannabidiol In Neurological Diso...
- – A0580 - Sigma-aldrich now available in LA
Latest Posts
Chest Strap Vs Wristband Heart Rate Monitors - Breaking Muscle now in NC
Which Heart Rate Monitor Is Best For Your Clients? - Total Gym now in TEXAS - limited time
Best Fitness Trackers 2023 - Live Science now in LA
Navigation
Latest Posts
Chest Strap Vs Wristband Heart Rate Monitors - Breaking Muscle now in NC
Which Heart Rate Monitor Is Best For Your Clients? - Total Gym now in TEXAS - limited time
Best Fitness Trackers 2023 - Live Science now in LA