Table of Contents
A Nonconventional Approach To Parkinson's Disease Therapy now in TEXAS

Results: Fifteen participants enrolled, two were screen failures. All 13 participants (10 male), mean (SD) age 68. 15 (6. 05), with 6. 1 (4. 0) years of PD, reported adverse events, including diarrhea (85%), somnolence (69%), fatigue (62%), weight gain (31%), dizziness (23%), abdominal pain (23%), and headache, weight loss, nausea, anorexia, and increased appetite (each 5%).
Elevated liver enzymes, mostly a cholestatic pattern, occurred in five (38. 5%) participants on 20-25 mg/kg/day, only one symptomatic. Three (23%) dropped out due to intolerance. Ten (eight male) that completed the study had improvement in total and motor Movement Disorder Society Unified Parkinson Disease Rating Scale scores of 7.
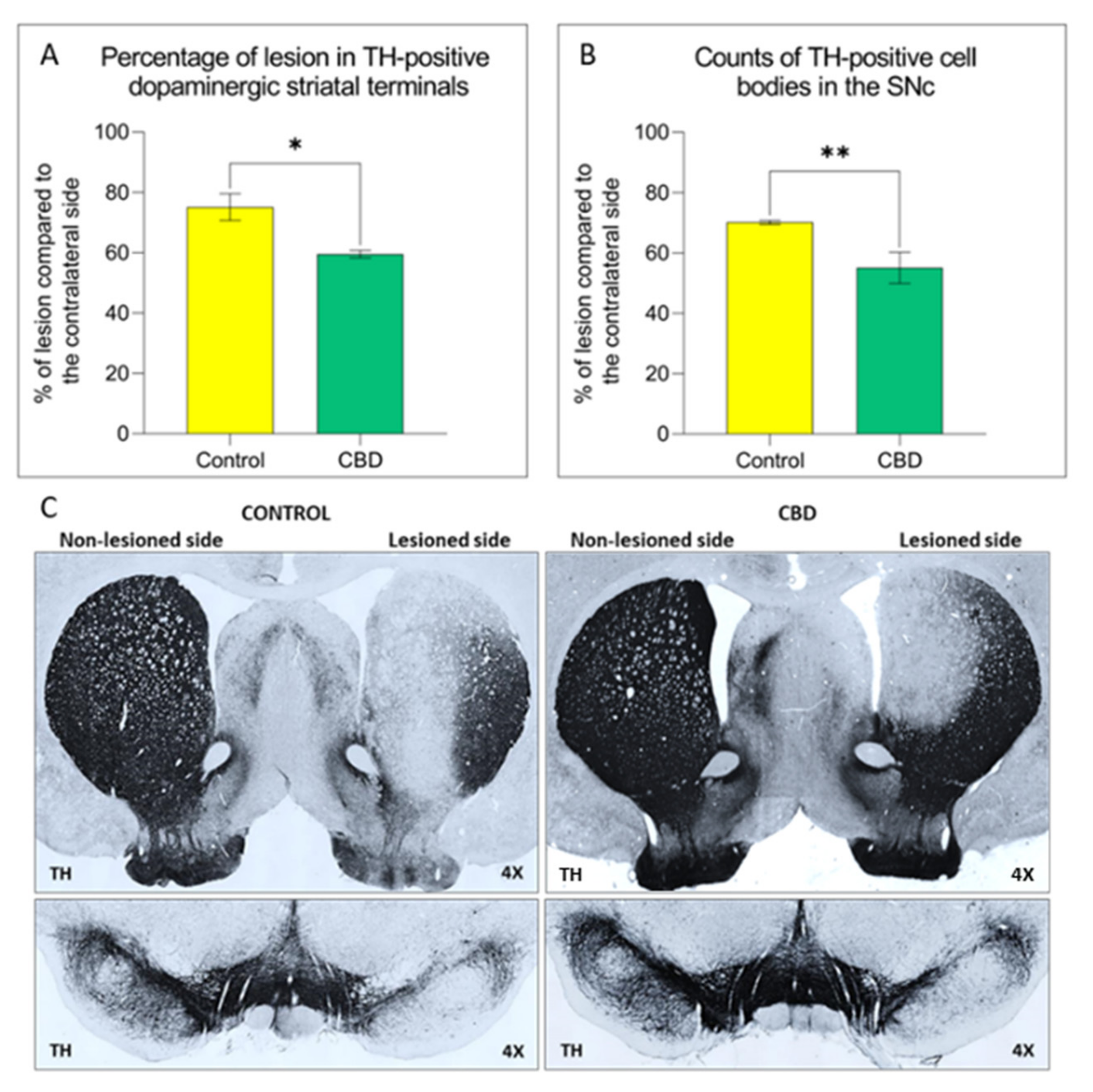
39, mean decrease 17. 8%, p=0. 012) and 6. 10 (6. 64, mean decrease 24. 7%, p=0. 004), respectively. Nighttime sleep and emotional/behavioral dyscontrol scores also improved significantly. Conclusions: CBD, in the form of Epidiolex, may be efficacious in PD, but the relatively high dose used in this study was associated with liver enzyme elevations.
Parkinson's disease; adverse events; cannabidiol; cannabis; parkinsonism.
Buy Ability To Slow Disease Progression And Safety And ... in NYC - limited period only
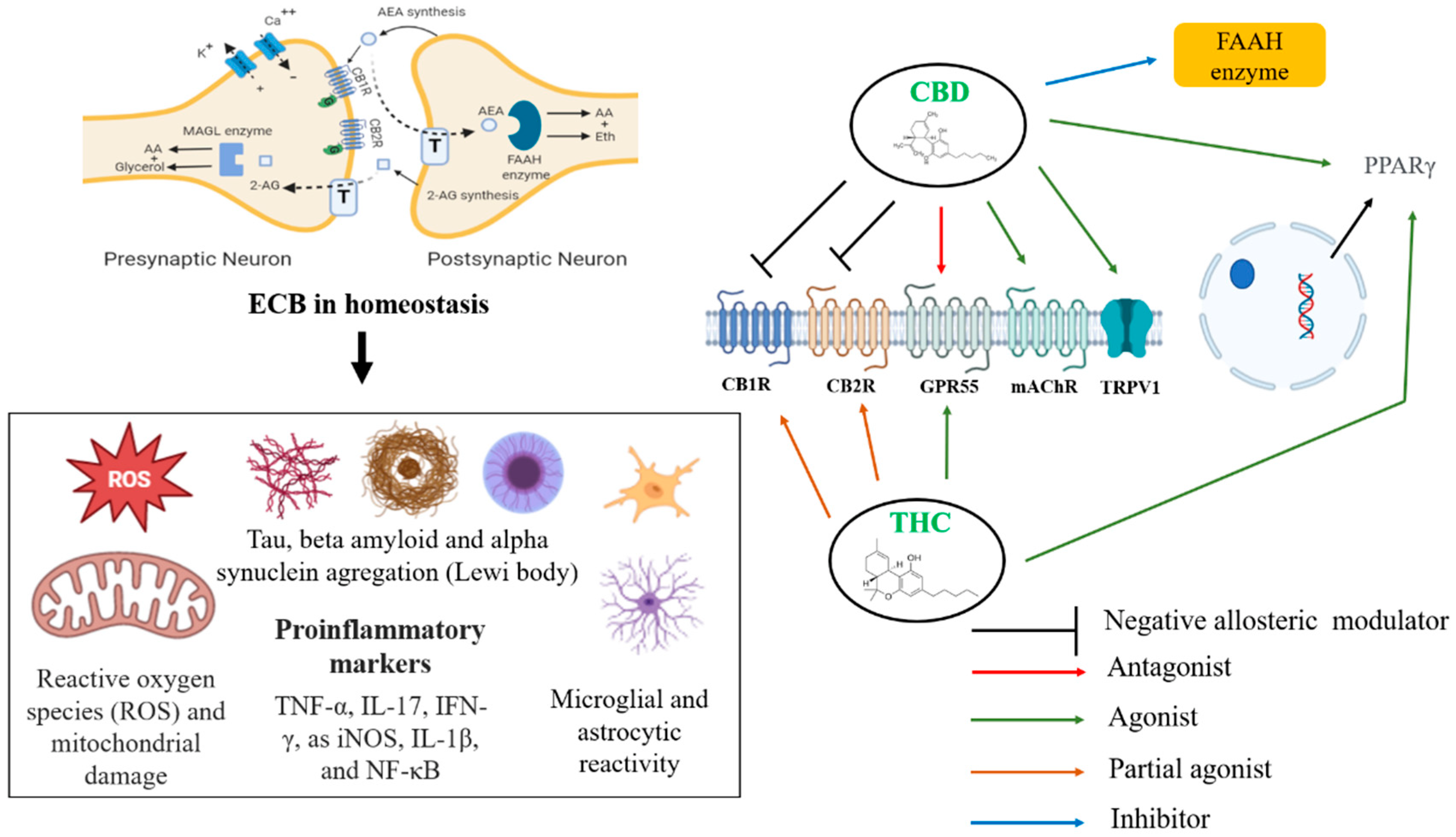
A prescription cannabidiol (CBD) oil is considered an effective anti-seizure medication. However, further research is needed to determine CBD's other benefits and safety. CBD is a chemical found in marijuana. CBD doesn't contain tetrahydrocannabinol (THC), the psychoactive ingredient found in marijuana that produces a high. The usual CBD formulation is oil, but CBD is also sold as an extract, a vaporized liquid and an oil-based capsule.
Currently, the only CBD product approved by the Food and Drug Administration is a prescription oil called Epidiolex. It's approved to treat two types of epilepsy. Aside from Epidiolex, state laws on the use of CBD vary. While CBD is being studied as a treatment for a wide range of conditions, including Parkinson's disease, schizophrenia, diabetes, multiple sclerosis and anxiety, research supporting the drug's benefits is still limited.
Though it's often well-tolerated, CBD can cause side effects, such as dry mouth, diarrhea, reduced appetite, drowsiness and fatigue. CBD can also interact with other medications you're taking, such as blood thinners. Another cause for concern is the unreliability of the purity and dosage of CBD in products. A recent study of 84 CBD products bought online showed that more than a quarter of the products contained less CBD than labeled.

If you plan to use products containing CBD, talk to your doctor. Sign up for free, and stay up to date on research advancements, health tips and current health topics, like COVID-19, plus expertise on managing health. Learn more about Mayo Clinic’s use of data. To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you.
Buy A Nonconventional Approach To Parkinson's Disease Therapy in LA
If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Please, try again in a couple of minutes Retry Dec. 06, 2022 Show references Miller B. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708. FDA approves first drug compromised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. U.S. Food and Drug Administration. Accessed Nov.
State medical marijuana laws. National Conference of State Legislatures. http://www. ncsl.org/research/health/state-medical-marijuana-laws. aspx#2. Accessed Nov. 27, 2018. Devinsky O, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut Syndrome. The New England Journal of Medicine. 2018;378:1888. Cannabidiol. Natural Medicines. https://naturalmedicines. therapeuticresearch.com. Accessed Nov. 5, 2018. Cannabidiol. Facts & Comparisons e, Answers.
wolterskluwercdi.com/facts-comparisons-online/. Accessed Nov. 5, 2018. Portenoy RK, et al. Cancer pain management: Adjuvant analgesics (coanalgesics). https://www. uptodate.com/contents/search. Accessed Nov. 14, 2018.
Buy Safety Of Low Dose Cannabidiol in NYC
The investigational product (ZTL-103) was a formulation of 10 mg THC/10 mg CBD per ml oral solution. The product was manufactured under supervision of the trial sponsor by Replek Farm, Macedonia in accordance with Good Manufacturing Practice (GMP), International Council for Harmonisation-GMP (ICH-GCP), and local regulatory requirements. The product was packaged into ready-to-use pre-filled syringes by Suda Pharmaceuticals (Australia), under GMP conditions.
The study was conducted in accordance with the Helsinki Declaration of 1994 and subsequent amendments. The trial was prospectively registered with the Australian New Zealand Clinical Trials Register (CT-2019-CTN-01224-1). Eligible participants were aged over 25 years with CNCP, on long-term treatment (at least 12 months) with high dose (oral morphine equivalent daily dose [OMEDD] ≥ 60 mg) opioid analgesia.
Table of Contents
Latest Posts
Chest Strap Vs Wristband Heart Rate Monitors - Breaking Muscle now in NC
Which Heart Rate Monitor Is Best For Your Clients? - Total Gym now in TEXAS - limited time
Best Fitness Trackers 2023 - Live Science now in LA
Navigation
Latest Posts
Chest Strap Vs Wristband Heart Rate Monitors - Breaking Muscle now in NC
Which Heart Rate Monitor Is Best For Your Clients? - Total Gym now in TEXAS - limited time
Best Fitness Trackers 2023 - Live Science now in LA